Final Research Plan
Lipid Disorders in Children and Adolescents: Screening
January 15, 2015
Recommendations made by the USPSTF are independent of the U.S. government. They should not be construed as an official position of the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
The final Research Plan is used to guide a systematic review of the evidence by researchers at an Evidence-based Practice Center. The resulting Evidence Review will form the basis of the USPSTF Recommendation Statement on this topic.
The draft Research Plan was available for comment from January 23 until February 19, 2014 at 5:00 p.m., ET.
The final research plan on screening for dyslipidemia in children and adolescents is now divided into two reviews in response to public comments. One evidence review will address screening for familial hypercholesterolemia; the other review will address screening for multifactorial dyslipidemia.
Screening for Familial Hypercholesterolemia in Children and Adolescents
*Intermediate outcomes include lipid levels (total and LDL cholesterol) and atherosclerosis markers (carotid intima–media thickness, calcium score, pathological findings).
Screening for Multifactorial Dyslipidemia in Children and Adolescents
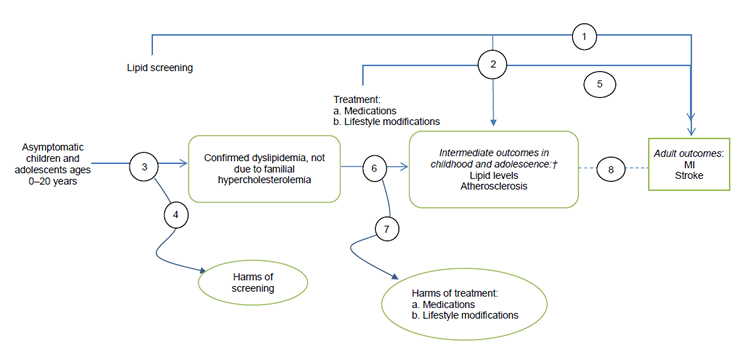
†Intermediate outcomes include lipid levels (total, LDL, HDL, and non-HDL cholesterol; triglycerides) and atherosclerosis markers (carotid intima–media thickness, calcium score, pathological findings).
Abbreviations: HDL = high-density lipoprotein; LDL = low-density lipoprotein; MI = myocardial infarction.
These figures depict the two analytic frameworks that outline the evidence areas covered in the research plan. In the first analytic framework, the population includes asymptomatic children and adolescents ages 0 to 20 years. After cholesterol screening (selective or universal), patients with familial hypercholesterolemia are identified. A separate arrow assesses the harms of screening. A subsequent branch leads to treatment (medications, lifestyle modifications), and an arrow from treatment assesses resulting harms. Adult health outcomes include myocardial infarction and stroke, and child and adolescent intermediate outcomes include lipid levels and atherosclerosis. In the second analytic framework, the same population is screened for dyslipidemia not due to familial hypercholesterolemia. Treatment and outcomes are the same as in the first analytic framework.
Screening for Familial Hypercholesterolemia in Children and Adolescents
- Does screening for familial hypercholesterolemia in asymptomatic children and adolescents delay or reduce the incidence of myocardial infarction (MI) or stroke in adulthood?
- Selective screening based on family history
- Universal screening
- Does screening for familial hypercholesterolemia in asymptomatic children and adolescents improve intermediate outcomes* (i.e., reduce lipid levels or reverse or slow the progression of atherosclerosis) in childhood and adolescence?
- Selective screening based on family history
- Universal screening
- What is the diagnostic yield of appropriate screening tests for familial hypercholesterolemia in children and adolescents?
- Selective screening based on family history
- Universal screening
- What are the harms of screening for familial hypercholesterolemia in children and adolescents?
- Does treatment of familial hypercholesterolemia with lifestyle modifications and/or lipid-lowering medications in children and adolescents delay or reduce the incidence of adult MI and stroke events?
- Does treatment of familial hypercholesterolemia with lifestyle modifications and/or lipid-lowering medications in children and adolescents improve intermediate outcomes* (i.e., reduce lipid levels or reverse or slow the progression of atherosclerosis) in childhood and adolescence?
- What are the harms of treatment of familial hypercholesterolemia with medications in children and adolescents?
- What is the association between intermediate outcomes* in childhood and adolescence and future incidence or timing of adult MI and stroke events?
*Intermediate outcomes include lipid levels (total and low-density lipoprotein cholesterol) and atherosclerosis markers (carotid intima–media thickness, calcium score, pathological findings).
Screening for Multifactorial Dyslipidemia in Children and Adolescents
- Does screening for multifactorial dyslipidemia† in asymptomatic children and adolescents delay or reduce the incidence of MI or stroke in adulthood?
- Does screening for multifactorial dyslipidemia in asymptomatic children and adolescents improve intermediate outcomes‡ (i.e., improve lipid levels or reverse or slow the progression of atherosclerosis) in childhood and adolescence?
- What is the diagnostic yield of screening for multifactorial dyslipidemia in children and adolescents?
- What are the harms of screening for multifactorial dyslipidemia in children and adolescents?
- Does treatment of multifactorial dyslipidemia with lifestyle modifications and/or lipid-lowering medications in children and adolescents delay or reduce the incidence of adult MI and stroke events?
- Does treatment of multifactorial dyslipidemia with lifestyle modifications and/or lipid-lowering medications in children and adolescents improve intermediate outcomes‡ (i.e., reduce lipid levels or reverse or slow the progression of atherosclerosis) in childhood and adolescence?
- What are the harms of treatment of multifactorial dyslipidemia with lifestyle modifications and/or lipid-lowering medications in children and adolescents?
- What is the association between intermediate outcomes‡ in childhood and adolescence and future incidence of adult MI and stroke events?
† Multifactorial dyslipidemia is defined as dyslipidemia not due to familial hypercholesterolemia, which is addressed separately.
‡ Intermediate outcomes in childhood and adolescence include lipid levels (total, low-density lipoprotein, high-density lipoprotein, and non–high-density lipoprotein cholesterol; triglycerides) and atherosclerosis markers (carotid intima–media thickness, calcium score, pathological findings).
Contextual questions are not systematically reviewed and are not shown in the Analytic Framework.
- Does initiation of statin treatment of familial hypercholesterolemia in childhood or adolescence result in better adult outcomes (delayed or reduced incidence of MI and stroke) than initiation in adulthood?
- How accurate is family history screening in identifying children and adolescents who are at higher risk for familial hypercholesterolemia?
The Research Approach identifies the study characteristics and criteria that the Evidence-based Practice Center will use to search for publications and to determine whether identified studies should be included or excluded from the Evidence Review. Criteria are overarching as well specific to each of the key questions (KQs).
| Included | Excluded | |
|---|---|---|
| Screening for Familial Hypercholesterolemia in Children and Adolescents | ||
| Population | KQs 1–4: Asymptomatic children and adolescents ages 0 to 20 years at time of screening
KQs 5–7: Children and adolescents ages 0 to 20 years at time of treatment initiation with a diagnosis of familial hypercholesterolemia KQ 8: Children and adolescents ages 0 to 20 years at beginning of study period with a diagnosis of familial hypercholesterolemia |
KQs 1–4: Children and adolescents with any of the following:
|
| Diseases | KQs 5–7: Familial hypercholesterolemia | KQs 5–7:
|
| Screening interventions | KQs 1–4:
|
KQs 1–4:
|
| Treatments | KQs 5–7:
|
KQs 5–7:
|
| Outcomes | KQs 1, 5, 8:
KQs 2, 6:
KQ 3:
KQ 4: All harms (e.g., false-positive or false-negative results, psychosocial effects, overdiagnosis) KQ 7: All harms from lipid-lowering medications (e.g., adverse events, long-term safety, overtreatment) |
KQs 1, 5, 8:
|
| Study design | KQs 1–3: RCTs, CCTs, cohort studies, systematic reviews
KQs 4, 7: RCTs, CCTs, cohort studies, systematic reviews, observational studies, systematically selected case series KQs 5, 6: RCTs, systematic reviews KQ 8: RCTs, CCTs, cohort studies, systematic reviews, registry studies, long-term trial followup, high-quality case-control studies |
KQs 1–3: RCTs, CCTs, cohort studies, systematic reviews
KQs 4, 7: RCTs, CCTs, cohort studies, systematic reviews, observational studies, systematically selected case series KQs 5, 6: RCTs, systematic reviews KQ 8: RCTs, CCTs, cohort studies, systematic reviews, registry studies, long-term trial followup, high-quality case-control studies |
| Settings |
|
Settings not generalizable to primary care |
| Screening for Multifactorial Dyslipidemia in Children and Adolescents | ||
| Population | All KQs: Children and adolescents ages 0 to 20 years
KQs 1–4: Asymptomatic children and adolescents ages 0 to 20 years KQs 5–8: Children and adolescents ages 0 to 20 years with dyslipidemia not due to familial hypercholesterolemia |
KQs 1–4: Children and adolescents with any of the following:
|
| Diseases | KQs 5–8: Dyslipidemia, as defined by the National Cholesterol Education Program (i.e., ≥1 of the following lipid/lipoprotein values, measured either in a nonfasting state followed by fasting or by 2 fasting tests):
Abnormal values are >95th percentile, except for HDL cholesterol, which is <10th percentile. Non-HDL cholesterol includes LDL cholesterol, lipoprotein-a, intermediate-density lipoprotein, and very-low density lipoprotein |
KQs 5–8:
|
| Screening interventions | KQs 1–4:
|
KQs 1–4:
|
| Treatments | KQs 5–7:
|
KQs 5–7: None
|
| Outcomes | KQs 1, 5, 8:
KQ 3:
KQ 4: All harms (e.g., false-positive or false-negative results, psychosocial effects, overdiagnosis) KQs 2, 6:
KQ 7: All harms from:
|
KQs 1, 5, 8:
|
| Study design | KQs 1, 2: RCTs, CCTs, and systematic reviews
KQ 3: RCTs, CCTs, systematic reviews, and cohort studies KQs 4, 7: RCTs, CCTs, systematic reviews, cohort studies, observational studies, systematically selected case-series, and qualitative studies KQs 5, 6: RCTs and systematic reviews KQ 8: RCTs, CCTs, systematic reviews, cohort studies, registry studies, long-term trial followup, high-quality case-control studies |
All KQs: Studies rated as poor quality
KQs 1–3, 5, 6, 8: Qualitative studies, case reports, cost-effectiveness studies KQs 1, 2, 5, 6: Cohort studies KQs 4, 7: None |
| Settings |
|
Settings not generalizable to primary care |
*Secondary causes of dyslipidemia include: renal (chronic renal disease, hemolytic uremic syndrome, nephrotic syndrome); infectious (acute viral or bacterial infections, HIV, hepatitis); hepatic (obstructive liver disease, cholestasis, biliary cirrhosis, Alagille syndrome); inflammatory (systemic lupus erythematosus, juvenile rheumatoid arthritis); storage (glycogen storage disease, Gaucher disease, cystine storage disease, Tay-Sachs disease, Niemann-Pick disease); and other (Kawasaki disease, anorexia nervosa, cancer, previous solid organ transplant, progeria, idiopathic hypercalcemia, Klinefelter syndrome, Werner syndrome, polycystic ovary syndrome, type 1 or 2 diabetes).
Abbreviations: CCT = controlled clinical trial; LDL = low-density lipoprotein; HDL = high-density lipoprotein; MI = myocardial infarction; RCT = randomized, controlled trial.
The draft research plan on screening for dyslipidemia in children and adolescents was posted for public comment from January 23 through February 19, 2014. In response to public comments, the evidence review was divided into two reviews—one addressing screening for familial hypercholesterolemia and the other addressing multifactorial dyslipidemia (defined as any dyslipidemia not due to familial hypercholesterolemia). The inclusion and exclusion criteria for the research approach to screening for familial hypercholesterolemia were clarified to explain that the review will exclude cascade screening or screening based on family history. Intermediate health outcomes for both topics were revised to include atherosclerosis as assessed via pathological studies.
